Legal procedures of biocidal products
The approval process for biocidal products consists of two legally required stages. A biocidal product can only be authorised when the active substance it contains is authorised.
Stage 1: The active biocidal substance is authorised as part of a European procedure (EU active substance review).
Stage 2: After the active substance has been authorised as per stage 1, biocidal products that contain the active substance can be approved in an approval procedure.
European procedures
As the basis for the national approval of biocidal products, fundamental decisions are first made at EU level which then apply in all EU member states. These decisions stipulate which active substances in biocidal products may be used in Europe (EU active substance review).
EU active substance review for authorization of active substances
The EU active substance review aims to establish a consistently high protection level for the health of humans, animals, groundwater and the environment in all member states and to reduce distortions of competition and trade barriers between individual member states caused by different approval practices. Only authorized active substances can be used in Europe as active substances in biocidal products
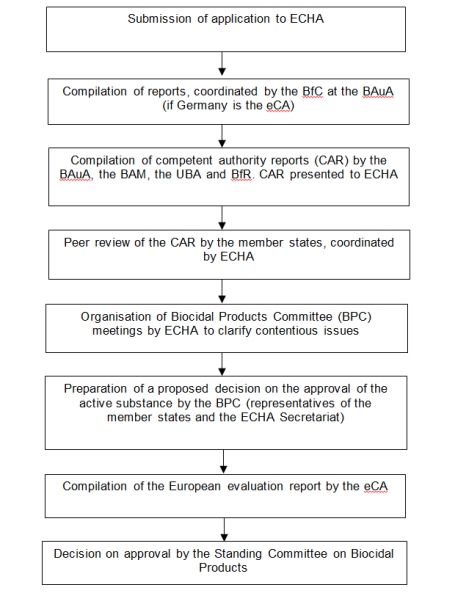
In the EU active substance review, one member state first tests and evaluates all information presented by the applicant. The evaluating competent authority (eCA) of this Member State produces an assessment report which is then presented to all other member states for peer review via the European Chemicals Agency (ECHA) and discussed at a meeting of experts, the Biocidal Products Committee (BPC). The final decision on the authorization of an active substance is made by the Standing Committee on Biocidal Products which is made up of representatives from the member states. Authorisation is generally valid for a period of ten years, after which the active substance is reviewed again at EU level.
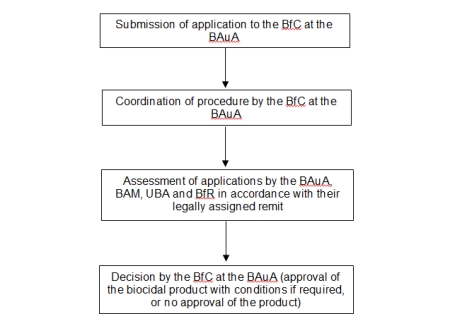
In Germany, the active substance review involves the same authorities as the national approval procedure. The tasks are coordinated by the Federal Office for Chemicals (BfC) located at the Federal Institute for Occupational Safety and Health (BAuA). BfR compiles and comments on the assessments in the following areas:
- Toxicology of the active substance and an exemplary biocidal product
- Exposure and risk characterisation of non-professional users, consumers and uninvolved third parties
- Residues in foods of plant or animal origin
- Analysis methods for monitoring residues in food, bodily fluids and the environment
Diagram of EU review process for active substances
National approval procedures
BfR participates in the following national procedures for biocidal products:
- National approval procedures for a biocidal product
- Mutual recognition of a national approval (from another member state of the EU)
- Union authorisation procedure
- Extension of an approval
For biocidal products containing active substances that were already on the market in Germany before EU Directive 98/8/EC (since replaced) and for which no authorization has yet been made, transitional provisions apply so that the product can remain on the market until a decision is made. These provisions require the notification of the biocidal product to the Federal Institute for Occupational Safety and Health (BAuA) in accordance with the German Ordinance on the impelmentation of Biocide Law (ChemBiozidDV). BfR is not involved in this process.
Approval procedure / Mutual recognition
Biocidal products consist of one or more active substances and so-called co-formulants (such as solvents). During the approval process, companies who wish to place biocidal products on the market must submit applications for approval. As a requirement, the active substances in these biocidal products must be authorized in the EU. Alternatively, companies can apply for recognition of an approval that has been granted by another EU member state. As part of this process, BfR may specify changes to the content of the approval due to country-specific regulations or legislation in order to ensure a higher level of consumer protection in Germany.
In Germany, the approval of biocidal products is carried out by the Federal Office for Chemicals (BfC), located at the Federal Institute for Occupational Safety and Health (BAuA), in cooperation with the Federal Environment Agency (UBA), the Federal Institute for Materials Research and Testing (BAM) and the Federal Institute for Risk Assessment (BfR), which each carry out partial assessments as part of their legally assigned remit. They review the information submitted by the companies in their applications, taking into account the efficacy, toxicology, potential residues and environmental behaviour of the biocidal products. BfR assesses the health risks to non-professional users, uninvolved third parties and consumers and also ensures that suitable analysis methods are available to monitor residues of the biocidal product in foods, bodily fluids and the environment.
If the authorities have no concerns, then the biocidal products are approved for the proposed applications.
Union approval
Union approval is a new procedure that was introduced when the Biocidal Products Regulation (EU) No 528/2012 came into force on 1 September 2013. The Union approval granted in this procedure applies throughout the European Union unless otherwise specified. In Chapter 8, Articles 41 to 46 of the Regulation, the conditions for granting and extending Union approvals are laid out. Applications are submitted to the European Chemicals Agency (ECHA), as is the case with the EU active substance review. If Germany is responsible for carrying out the assessment on behalf of the EU, the process is coordinated by the BfC (located at the BAuA) in cooperation with the same authorities as the national approval procedure or the EU review programme for active substances.
Simplified approval procedure
In another procedure regulated by Chapter 5, Articles 25 to 28 of the Biocidal Products Regulation (EU) No 528/2012, an application for approval can be made as part of a simplified approval procedure for certain eligible biocidal products. This procedure has been established since 2013. Biocidal products suitable for simplified approval are those whose active substances are listed in Article 25, Annex I of the Biocidal Products Regulation (EU) No 528/2012. A further requirement is that the biocidal product contains no substance of concern and no nanomaterials. Furthermore, the biocidal product must be sufficiently effective. Handling of the biocidal product and its intended use must also require no personal protective equipment. If Germany is responsible for carrying out the assessment on behalf of the EU, the procedure is coordinated by the BfC (located at the BAuA) in cooperation with the same authorities as the national procedures or the evaluation of active substances within the EU.
Diagram of national procedures