Risk assessment of biocidal products
Basis of risk assessment
For a biocidal product to be approved, the health of all groups of people who come into contact with the product or its residues must be ensured when the product is used correctly and in line with its intended purpose. In order to achieve this, toxicological effects must be identified and quantified. Furthermore, for each group of people who may be exposed to the biocidal product, the amount of the product they can come into contact with must be estimated (exposure).
The risk is determined by comparing the toxic effect with the exposure. This is illustrated in the following diagram.
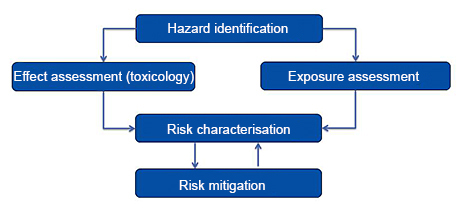
Identification of toxic effects
In order to ensure that the application of biocidal products does not pose an unacceptable risk to humans and animals, the products and the active substances they contain are examined and toxicologically assessed by BfR. This takes place before approval.
Biocidal products can be made up of a variety of components:
- Active substance(s); finished biocidal products usually contain one active substance; in individual cases, as many as four active substances may be present.
- Various co-formulants such as preservatives, antioxidants, anti-foam agents, emulsifiers, and solvents.
The scope of toxicological analyses for biocidal products is regulated by law.
Active substances which are to be used in biocidal products must undergo very extensive tests for potential adverse health effects:
- Toxicokinetics and metabolism
- Acute toxicity, skin and eye irritation, sensitisation
- Subchronic toxicity (toxicity after repeated dose exposure)
- Genotoxicity
- Chronic toxicity (long-term toxicity)
- Carcinogenicity (cancer-causing potential)
- Reproductive and developmental toxicity
- Neurotoxicity
- Endocrine disrupting properties
- Additional studies if necessary, e.g. on toxicological mechanisms of action
Biocidal products are additionally tested for the following effects:
- Acute toxicity (oral, dermal, inhalation)
- Irritation of skin and eyes, skin sensitisation
- Dermal absorption (intake through the skin)
Derivation of limit values
Dose-response relationships are derived for the detected toxic effects. It is assumed that the majority of toxic effects are subject to a threshold value – in other words, an adverse health effect only occurs if a certain dose (threshold) is exceeded.
The NOAEL (no observed adverse effect level) serves as the basis for the determination of limit values. This is the dose at which no adverse health effects were observed in experimental studies. The NOAEL taken from a study on rats, for example, is divided by a(n) (un)certainty factor which not only accounts for the different sensitivities of humans and animals, but also the different sensitivities of individuals and therefore particularly sensitive groups of people such as children, pregnant women or sick people. A factor of 100 is usually used for this. As long as exposure is not above the limit values calculated in this way, it is assumed that there is no health risk to users, uninvolved third parties of consumers.
The following limit values are usually derived for active substances of biocidal products:
- AEL stands for "acceptable exposure level" and represents a limit value for the exposure of users of biocidal products and uninvolved third parties. These are people who may come into direct contact with the biocidal product during or after use. AELs are derived for three exposure periods: for one-time (acute) exposure, for medium-term, multiple exposure, and for long-term exposure.
- ADI stands for "acceptable daily intake" and specifies the amount of a substance that consumers can ingest with food on a daily basis over a lifetime without any discernible health risk. The ADI represents a limit value for the long-term exposure of consumers.
- ARfD stands for "acute reference dose" and specifies the amount of a substance that consumers can ingest with food over one or more meals in the course of one day without any discernible health risk. The ARfD represents a limit value for the short-term exposure of consumers.
- It can be assumed that certain toxic effects are not subject to a threshold, meaning no exposure level can be identified that does not have a harmful effect. In the biocide procedure, no limit values are derived for substances with these toxic effects. In particular in the case of carcinogenic substances that exhibit a genotoxic mechanism of action without a threshold value, BfR does not support approval of the corresponding biocidal products.
Exposure assessment
Along with data on toxicological effects, data regarding the exposure of the groups of people in question to biocidal products during or after their application in line with their intended purpose are required in order to assess a health risk. For this purpose, BfR determines the amounts of biocidal products or their residues that can be ingested by consumers. The fact that different groups of people can be exposed in very different ways with regard to intake path, duration and level of exposure is taken into account. During the application of biocidal protection products, such as insect spray, users or other people who are in the room may inhale the product or absorb it through their skin. Consumers may also ingest residues of biocidal products via food (orally).
