Dermatotoxicology Study Centre
Foundation
The Dermatotoxicology Study Centre was established in February 2021. Our work focuses on the sub-areas ‘allergy’ and ‘tattoo inks’.
Objectives
The core competence of the Dermatotoxicology Study Centre is interdisciplinary research bridging analytics, toxicology, immunology, and clinical research to investigate the health hazards of chemicals in consumer-related products such as jewellery, cosmetics, and tattoo inks (Fig. 1). Our research is helping to reduce animal experiments in the long term and improves risk assessment. These goals can only be achieved if the molecular and cellular responses to chemicals are as well understood as possible.
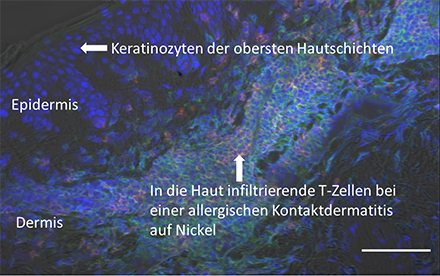
Fig. 1: Allergic contact dermatitis
The image shows the infiltration of T cells into the skin. Usually, T cells fight pathogens such as viruses or bacteria. Here, an allergic reaction to nickel is depicted. T cells are the main players during the elicitation of contact allergy and orchestrate the ensuing immune responses. In the case of allergic reactions to tattoos, the T cell infiltration looks similar. Scale 100 µm. Blue - DAPI (nuclear staining), green - CD3 (T cells), red - CD8 (cytotoxic T cells).
Toxic effects of tattoo inks
Because tattoos are introduced into the central, dermal layer of the skin, exposure is not comparable to conventional routes of administration (i.e. oral, dermal or inhalative uptake).
In January 2021, the European Commission published a Europe-wide restriction on tattoo inks under the REACH legislation in cooperation with the German Federal Institute for Risk Assessment. However, the current data situation does not yet allow for a robust risk assessment of the various tattoo inks. The aims of the Dermatotoxicology Study Centre are:
- to fill data gaps
- to develop and apply test strategies to determine certain toxic effects
- to use these methods to identify possible adverse effects (incl. the involved pathomechanisms)
T cell activation by sensitising chemicals
T cells are causally involved in triggering allergic contact dermatitis. However, there is not validated in-vitro-test for this final key event of skin sensitisation. The Dermatotoxicology Study Centre investigates chemical and product-induced allergies with a focus on the development of T cell-based predictive and diagnostic in-vitro-tests, which replicate the processes in human skin. The development of such tests is an essential prerequisite for a better understanding of the disease and the sensitising properties of chemicals and products. We also focus on the elucidation of possible cross-reactivity reactions to structurally related chemicals.
Methods
The Dermatotoxicology Study Centre applies and further develops state of the art methods. These currently include the development of immunocompetent skin models with integrated tattoo ink pigments, analytical methods such as high-resolution mass spectrometry, activation-induced marker (AIM) assays for the detection of antigen-specific T cells, multiparameter flow cytometry, single cell sorting, as well as high-throughput sequencing technologies. Furthermore, we conducts studies with patient samples like blood, urine, and skin tissue samples from allergic and non-allergic patients within the framework of our extensive clinical collaborations.
Publications
Complete lists of publications at:
- https://orcid.org/0000-0003-0380-1594 (Siewert)
- https://orcid.org/0000-0001-9313-7193 (Schreiver)
