Questions and answers on the notification of dangerous products for emergency medical care in Germany for notifiers according to Art. 45 of the CLP Regulation
Complete revision of the version dated 23 December 2020. The individual topics were regrouped and supplemented with questions from the 14th BfR user conference on product notifications.
The German Federal Institute for Risk Assessment (BfR) receives product notifications from industry. The BfR checks these product notifications and makes them available to the poison centres of the federal states for emergency medical advice.
Background: Chemical products are usually complex mixtures of various ingredients or components. Due to their hazard potential – especially if they are not used as intended – they can cause health risks for consumers. Persons and companies that place hazardous (chemical) mixtures on the market must provide the BfR with relevant information. The legal basis for this is Article 45 of the Regulation on classification, labelling and packaging of substances and mixtures (EC) No. 1272/2008 (CLP: classification, labelling and packaging), which is enshrined in national law via the Chemicals Act (Section 16e ChemG).
The BfR has compiled frequently asked questions on the notification of hazardous products in order to answer common and selected detailed questions on the subject of product notifications quickly and transparently. Additional information can be found in the linked sources.
Safety data sheetWhich number can I enter as an emergency contact in section 1.4 of the safety data sheet (SDS)?
You can organise an emergency call service yourself or commission one of the poison centres (for a fee) to do so. Accordingly, enter the telephone number of your own service or the phone number of the commissioned poison centre in section 1.4 of the SDS. Entering a telephone number for the BfR or the fire brigade is not correct, as neither the BfR nor the fire brigade offer an emergency advice service.
Legal basis:
- Annex II, section 1.4, of the REACH Regulation (EC) No 1907/2006
Further information:
Why can't I specify the BfR as an emergency contact in section 1.4 of the safety data sheet (SDS)?
The REACH Regulation states: “If there is a public advisory body [...] in the Member State [...], its telephone number shall be given, which may be sufficient.”
Legally speaking, no such “public advice centre” exists in Germany. The BfR is also not a “public advice centre” and may not be named as an emergency contact. Furthermore, the BfR does not provide emergency counselling and is not a suitable contact for this reason alone.
Legal basis:
- Annex II, section 1.4, of the REACH Regulation (EC) No 1907/2006
Further information:
Does the emergency contact in section 1.4 of the safety data sheet (SDS) have to be available 24/7?
No, but in this case, you must specify the service times in the SDS.
Legal basis:
- Annex II, section 1.4, of the REACH Regulation (EC) No 1907/2006
Further information:
Can emergency health counselling also be provided in English?
The contents of the safety data sheet must be drawn up in the official language(s) of the member state. The purpose of this regulation is to ensure that the recipient understands the contents and can apply them for the safe use of the substance or mixture. The legal text does not make a specific statement on the language of the emergency information services, but according to the above-mentioned meaning and purpose, it can be assumed that the emergency advice must also be offered in the official language. For Germany, this is German.
Legal basis:
- Art. 31, para. 5 of the REACH Regulation (EC) No. 1907/2006
Further information:
UFIWhat is a UFI?
UFI stands for Unique Formula Identifier and is a unique 16-digit alphanumeric code that is linked to a formulation and the product(s) made from it via a product notification (dossier). It is a mandatory component of the notification of mixtures classified as hazardous in PCN format.
The UFI code itself (wherever it is used) must be preceded by the acronym “UFI:” in capital letters and must be clearly visible, legible and indelibly marked. The acronym “UFI:” must always be used in Latin characters followed by a colon, regardless of the country, language and national alphabet. The European Chemicals Agency (ECHA) provides the UFI generator, a free tool for creating one or more UFIs.
How do I create a UFI?
The European Chemicals Agency (ECHA) provides the UFI generator, a free tool for creating one or more UFIs. Alternatively, a UFI generation algorithm is also available for companies. This means that the UFI generator can be integrated into your own systems.
Further information:
Can I use different UFIs for a mixture that is marketed under different trade names and possibly by different companies?
Yes, a company can generate several UFIs for the same mixture that are placed on the market under the same or different trade names. The same UFI can also be used by different companies along the supply chain as long as the composition remains the same. There can therefore be one or more UFIs for the same formulation.
For example, a company can assign different UFIs according to market area, language or trade name.
How do I submit a UFI to the BfR?
The UFI is part of a product notification in PCN format. When creating a PCN notification, you can either assign a UFI for all products in a recipe or assign a separate UFI to each product.
When do I have to specify a new UFI for my product mixture in my product notification (PCN)?
When a product classified as hazardous is notified for the first time and after significant changes to the formulation of a product, a new UFI must be issued and submitted in a corresponding product notification. In both cases, a new PCN number (not the submission number) is also assigned. For product notifications with significant changes of composition, the PCN number of the previous product version must be specified in the dossier as the related PCN number.
Further information:
Obligation to notify in PCN format and transition periodsWhat must be reported and what not?
All mixtures that are classified as hazardous to health and physically dangerous are subject to notification.
Not subject to notification:
- Only environmentally hazardous mixtures
- Gases under pressure
- Explosive mixtures
- Mixtures for scientific research and development
Mixtures for process-orientated research and development
When am I obliged to create a product notification in PCN format?
The CLP Regulation distinguishes between product mixtures for private/commercial and for exclusively industrial use. Since 1 January 2024, all product notifications, i.e. initial notifications and updates, must be made in PCN format – a special format to notify poison centres (PCN: Poison Centres Notification). However, the following transitional arrangements apply: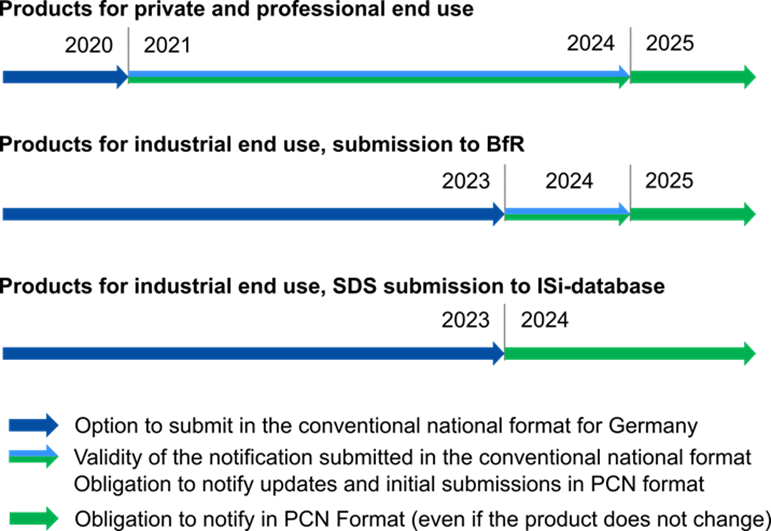
What notification channels are there for products classified as hazardous in Germany?
Germany accepts product notifications for products/mixtures classified as hazardous only in PCN format (i6z file). The European Chemicals Agency (ECHA) refers to a product notification as a dossier. There are two ways to submit a dossier:
- The notification via the Submission Portal of the ECHA. ECHA distributes the dossier to the member states specified in the dossier for commercialisation.
After submitting the dossier to the ECHA portal, you will receive a link to a dossier-specific Submission Status Page, which contains information on the status and success of your notification under Submission Events.
As soon as your notification has been received by the BfR, the notification obligation is formally fulfilled (Submission Event: Dossier received by DE) and no further confirmation is given by the BfR.
- For product notifications that only contain products for the German market, it is possible to submit the i6z file directly via the BfR portal.
For product notifications sent directly to the BfR, the BfR issues confirmation letters that confirm formal acceptance and are sent to the notifier.
Can the BfR still demand corrections after the formal acceptance of a product notification?
Yes, the BfR validates the content of all product notifications and, if necessary, requests information or corrections that it considers necessary for the fulfilment of its tasks.
Legal basis:
- Art. 45, para. 3 of the CLP Regulation (EC) No. 1272/2008
When is a contract manufacturer or rebrander/relabeller obliged to notify?
A contract manufacturer is always subject to notification according to Art. 45 CLP Regulation, he is the responsible distributor (submitter). A downstream relabeller/rebrander as a downstream user[1] is only subject to notification if he carries out one of the following activities:
- Relabelling
- Decanting
- Changing the outer packaging
If the relabeller/rebranding company is not subject to the notification obligation because it orders the product as it is placed on the market, the situation arises where the contract manufacturer is stated as the submitter in the PCN dossier, whereas the relabeller/rebranding company is stated on the product label. This is correct and means that the company on the label is not always the company subject to notification. The company name cannot therefore be used for identification – e.g. in the event of poisoning. The decisive factor is the product name in combination with the corresponding UFI.
[1] Some countries, including Germany, are of the legal opinion that labellers/rebrander become so-called downstream users and are therefore subject to notification under Art 45 of the CLP Regulation.
How does the product notification as a “Foreign User” take place on the ECHA Submission Portal?
Each company obliged to submit a notification (“submitter”) must create its own account on the ECHA Submission Portal in order to be able to submit the notification on its behalf.
If the notification is to be carried out by a third party, the so-called “foreign user”, on behalf of another company obliged to notify, the company must authorise the third party as a “foreign user” to access their account in order to carry out the notification.
The third party must also set up an account with ECHA and first logs in with their own account, then switches to the company's account and carries out the notification in their name. Only the company obliged to notify will then appear in the dossier.
Further information:
- PCN: A practical guide (describes, among other things, how to set up a “foreign user”)
How do I, as a “foreign user” (contract manufacturer), keep the formulation secret from the company obliged to notify (relabeller)?
Both companies require an ECHA account. The contract manufacturer must be set up as a “foreign user” by the relabeller. The contract manufacturer uses its own account to submit a product notification with the formulation details and UFI (if available). The contract manufacturer switches to the relabeller's account (“Switch legal entity”) and submits the product notification for the relabeller as 100% MiM of the product previously submitted by the relabeller.
Further information:
Where can I get information on how other countries participating in the PCN procedure handle product notifications?
Information on alternative notification routes, permitted languages, fees and earliest market placement can be found in the document Overview of Member States decisions on implementing Annex VIII to the CLP on the ECHA website.
The Notified Bodies (Appointed Bodies) of the participating countries, including their contact details, can also be found on this page.
Further information:
Combination of a mixture with other mixtures or productsHow do I submit a product that contains several mixtures that are separate from each other? (e.g. dishwasher tabs/pods, two-component adhesive)
Each mixture classified as hazardous to health or physically dangerous according to the CLP Regulation must be reported in a separate notification with a separate UFI.
Assign the same multi-component ID for the individual mixtures in the product notifications to indicate that they belong to an overall product.
Information on the mixture resulting from the use of the product is also of interest for emergency medical care and should be provided in the toxicology section of the safety data sheet, if applicable.
Further information:
- Guideline on harmonised information for emergency health response - Annex VIII to CLP Regulation, section 4.2.8.1 (multi-component products)
- Guidance on labelling and packaging according to Regulation (EC) No 1272/2008, section 6.2 (Specific case: labelling of two-component products)
How do I transfer a product mixture that is integrated into another product that is not a mixture? (e.g. ink pads, candles, wet wipes)
The other product is a so-called article. The guideline distinguishes between different cases. Please refer to the relevant section, as the content is too extensive to summarise here.
Further information:
- Guidance on harmonised information for emergency health response - Annex VIII to CLP, section 3.1.1.4 (import/manufacture of a mixture/product combination)
Voluntary product notification of non-hazardous productsHow do I submit a voluntary product notification in PCN format?
In PCN format, it is also possible to submit products that are not subject to mandatory notification, or contain only one component. To do this, mark your PCN notification under submission type as voluntary submission.
Further information:
How do I submit a voluntary product notification in XProductNotification format?
For voluntary product notifications of products not subject to mandatory notification, the BfR also accepts transmission via the national procedure in the XProductNotification format.
You will find the required forms on the BfR website under Notification of Hazardous Products in the right-hand column. ().
- Use the first form to apply for a company code once. To do this, fill out the form and send it to produkt-meldungen@bfr.bund.de. You will then receive your company code shortly. If you have already received a company code for your company, you can use it for the XProductNotification.
- The second file in zip format contains the files XProductNotification_BfR.xls (32-bit version) and XProductNotification_BfR_64_Bit.xlsm (64-bit version). Use one of these files to enter the data for your product(s). Always enter the company code as the company details. Then create the XML message by pressing the button and save it locally. A popup appears in which you enter the company code again.
- Then submit the XML file via the BfR portal . A prerequisite for using the BfR portal is a user account, which you create before uploading for the first time. This account is independent of the BfR company code and guarantees secure data transmission.
If the notification to the BfR is successful, you will receive a confirmation letter within two to four weeks of receipt.
Notes:
- In contrast to the PCN format, where you transmit a formulation and assign several products to this recipe, you transmit individual products with their formulation in the XProductNotification format.
- Even if the Unique Formula Identifier (UFI) is not part of the XProductNotification format, you can still assign a UFI to your products. To do this, enter the UFI in the Other Product Identification field of the Notification Part 4 worksheet of the Excel form in the form “UFI: xxxx-xxxx-xxxx-xxxx-xxxx” (without quotation marks). The BfR recommends the optional UFI entry, as it facilitates quick and reliable formulation assignment in the poison centre.
- The XProductNotification format is only applicable for submitting products for the German market.
Comparable forms are available for Openoffice.
When is a contract manufacturer or rebrander/relabeller obliged to notify?
A contract manufacturer is always subject to notification according to Art. 45 CLP Regulation, he is the responsible distributor (submitter). A downstream relabeller/rebrander as a downstream user[1] is only subject to notification if he carries out one of the following activities:
- Relabelling
- Decanting
- Changing the outer packaging
If the relabeller/rebranding company is not subject to the notification obligation because it orders the product as it is placed on the market, the situation arises where the contract manufacturer is stated as the submitter in the PCN dossier, whereas the relabeller/rebranding company is stated on the product label. This is correct and means that the company on the label is not always the company subject to notification. The company name cannot therefore be used for identification – e.g. in the event of poisoning. The decisive factor is the product name in combination with the corresponding UFI.
RecipeCan I combine perfumes into one entry in the formulation?
Product mixtures that differ only in the composition of their perfume substances can be summarised in a group notification with a generic component identifier (GCI) in one notification, which may be made under the following conditions:
- All product mixtures in the group contain the same qualitative composition (substances and concentrations) with the exception of the perfumes.
- The reported concentration or concentration range is the same for each component.
- All mixtures in the group have the same classification with regard to health and physical hazards (differences in the classification with regard to environmental hazards are permitted).
- The total concentration of perfumes does not exceed 5 %.
Further information:
How do I specify a product recipe if my supplier does not provide me with the complete recipe information?
In principle, it is possible to indicate a mixture as a formulation component instead of a substance (a so-called mixture in mixture, MiM) if the notifier does not have the complete formulation of an incorporated mixture. In principle, all available information on the formulation should be provided. This results in the following information that must be included in a MiM declaration:
If the MiM was communicated to the BfR in an earlier PCN notification:
- Product identifier (trade name or designation of the MiM)
- UFI
- Concentration
- Classification with regard to health and physical effects
If a UFI is available for the MiM, but the BfR has not received the information on the MiM in a previous PCN notification:
- Product identifier (trade name or designation of the MiM)
- UFI
- Concentration
- Classification with regard to health and physical effects
- Name, e-mail address and telephone number of the MiM supplier
- The compositional information contained in the MiM safety data sheet
- All other known components of the MiM
If there is no UFI for the MiM:
- Product identifier (trade name or designation of the MiM)
- Concentration
- Classification with regard to health and physical effects
- Name, e-mail address and telephone number of the MiM supplier
- The compositional information contained in the MiM safety data sheet
- All other known components of the MiM
Further information:
- CLP Regulation, Art. 18, para. 3a (Product identifiers)
- CLP Regulation, Annex VIII, Part B, Section 3.2.2
Notification procedure and costsHow much does a product notification cost for Germany?
All product notifications to the BfR are free of charge.
Can I send a product notification directly to the BfR or does this have to be done via the ECHA?
Product notifications in PCN format are distributed by ECHA to the countries according to the markets indicated.
However, Germany also permits the direct transmission of a product notification (as an i6z file) to the BfR if the product notification only contains products for the German market.
Will I receive a confirmation from the BfR that I have received the product notification?
After completing the notification via the ECHA portal, you will receive a link to a notification-specific Submission Status Page. Information on the status and success of your notification can be found there under Submission Events. The Submission Event Dossier received by DE is the formal confirmation of your product notification.
If you are using a commercial software product with System 2 System (S2S) submission of your product notifications to ECHA, there should be a comparable response in your software. If necessary, consult the manual or contact the manufacturer of your software product.
If you have sent a product notification in PCN format in the form of an i6z file directly to the BfR, you will receive a confirmation letter by post after checking. Find out about the conditions when the direct route to the BfR is permitted in the answer to the question "Can I send a product notification directly to the BfR or does it have to be sent via the ECHA?” of this FAQ.
Further information:
